Virus Production
Choose the type of recombinant virus that fulfill your needs and the VVPP can package it for you.
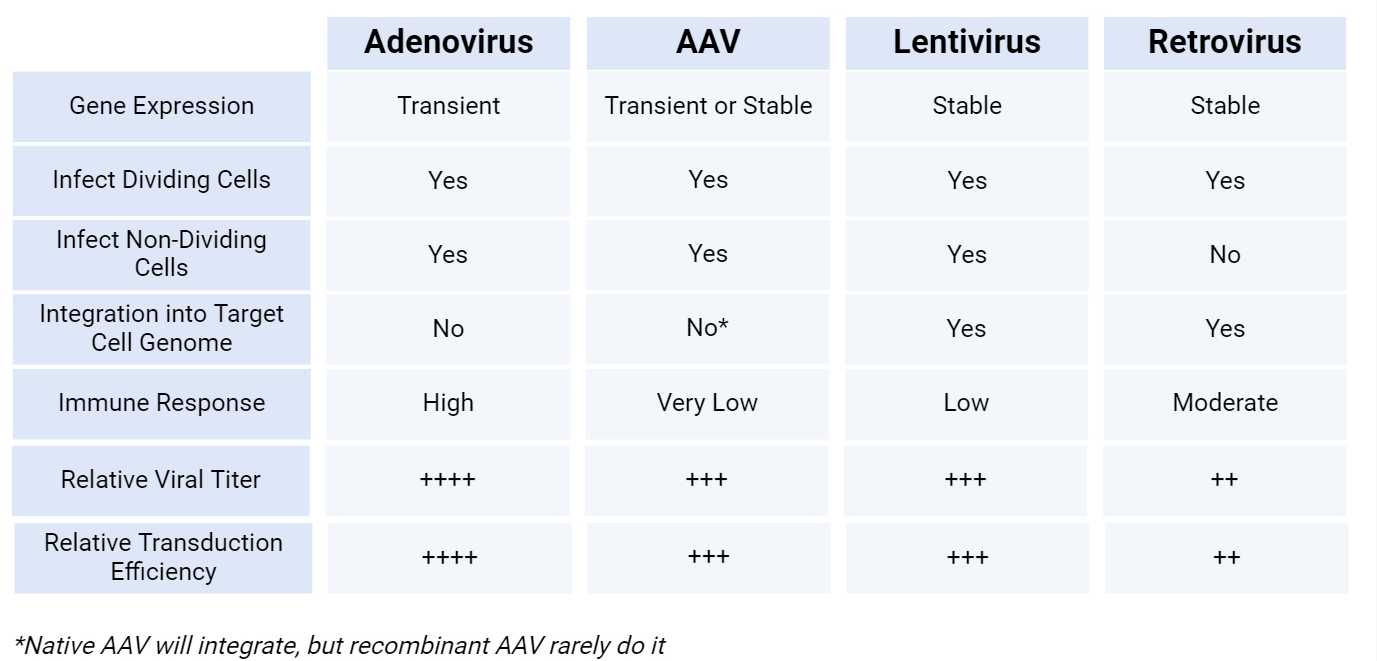
We offer Recombinant Moloney Murine Leukemia Viruses (MMLV) and Recombinant Murine Stem Cell Viruses (MSCV) to transduce/infect dividing cells for ORF and shRNA stable expression, both in vitro and in vivo. While MMLV can integrate into the target cell genome of a variety of dividing cells, especially iPS, MSCV serves mainly for robust expression of genes or shRNA into embryonic stem (ES) cells, hematopoietic stem (HS) cells, embryonal carcinoma (EC) cells. The presence of a strategically designed 5’LTR derived from the murine stem cell PCMV virus in the MSCV retroviral vector allows to overcome the restricted expression of ORF or shRNA in ES and EC cells, typically observed with MMLV retroviral vectors. For retrovirus manufacturing, we use Phoenix-ECO packaging cell lines (for transduction/infection of mouse and rat cells) or Phoenix-AMPHO packaging cell lines (for infection of mammalian cells). These cells stably express packaging genes (viral Gag-Pol) and the envelop gene (viral Env) eliminating the need to deliver these genes in trans by specific vectors. By this system, retroviruses are produced just in a few days following the transfection of the retrovirus vector carrying the ORF or shRNA for the gene of interest. After purification and concentration, retrovirus titer is established by HEK-293T cell transduction, gDNA extraction and qPCR analysis. Unless others that provide a physical titer, we deliver a biological titer, which reflects the real activity of the viral particles. The immune response to retrovirus is classified as moderate. Our retroviruses are bacteria, fungi, mycoplasma and endotoxin-free.

We offer recombinant lentiviruses to transduce/infect both dividing and not dividing cells for ORF and shRNA stable expression, both in vitro and in vivo. Unlike plasmid DNA vectors, which only allow transient and episomal expression of the foreign DNA sequence in the host cell, lentiviral vectors allow for permanent expression of the foreign DNA sequence by integration into the target cell genome.Lentiviruses are also efficient for in vivo gene delivery for the generation of transgenic animal models. For second and third-generation lentivirus manufacturing, we co-transfect into HEK-293T packaging cells the transfer vector carrying the ORF or shRNA for the gene of interest together with the packaging vector(psPAX2) encoding Gag/Pol and the envelop vector(pMD2.G) encoding VSV-G. After purification and concentration, lentivirus titer is established by HEK-293T cell transduction, gDNA extraction and qPCR analysis. Unless others that provide a physical titer, we deliver a biological titer, which reflects the real activity of the viral particles. The titer obtained with lentiviruses is generally higher when compared to retrovirus titers. The immune response to lentivirus is classified as low. The transduction/infection efficiency of lentiviruses is higher than retroviruses. Our lentiviruses are bacteria, fungi, mycoplasma and endotoxin-free.

We offer recombinant adenoviruses to transduce/infect both dividing and not dividing cells for ORF and shRNA transient expression in a wide variety of mammalian cell types, both in vitro and in vivo, where the adenovector remains as episomal DNA without integration into the host genome. High transduction/infection efficiency and high levels of short-term ORF/shRNA expression make adenoviral vectors a preferred tool when a greater gene overexpression or knock-down is required. Adenoviruses can be used not only for gene therapy, but also for vaccination because of their capacity to induced a high immune reaction in vivo. For adenovirus manufacturing, the adenoviral vector carrying the ORF or shRNA for the gene of interest is first linearized and then is transfected into HEK-293A packaging cells expressing the adenovirus gene E1 to produce recombinant adenovirus. The adenoviral particles are released into cell culture medium where they are collected as crude lysate, which can be used for in vitro experiments. For in vivo purified-grade adenovirus, crude lysate is used to transduce again the packaging cells. Adenoviral particles are then harvested from cell lysate, purified and concentrated. Unless others that provide a physical titer, we provide a biological titer, which reflects the real activity of the viral particles, by performing a plaque assay. The titer obtained from adenoviruses is generally higher than the titer obtained from lentiviruses and retroviruses, and the in vivo immune response is higher. The transduction/infection efficiency of adenoviruses is higher compared to lentiviruses or retroviruses. Our adenoviruses are bacteria, fungi, mycoplasma and endotoxin-free.


We offer recombinant adeno-associated viruses (AAV) to transduce/infect both dividing and not dividing cells for ORF and shRNA transient/stable expression, both in vitro and in vivo. Due to the broad choice of serotypes (different tropism for different tissues), AAV represent a versatile genetic tool for gene delivery. Also, AAV have emerged as one of the most effective vehicles for gene therapy in humans because of their very low immune-induced reaction in vivo. We can provide several AAV serotype (1,2,3,4,5,6,7,8,9, DJ, DJ8). Moreover, each serotype can be produced as ssAAV or scAAV. scAAV have half of the packaging capacity of ssAAV, but the transgene is expressed earlier and more robustly when compared to ssAAV. This make scAAV ideal for shRNA expression. For manufacturing AAV helper-free adenovirus, the transfer vector carrying the ORF or shRNA for the gene of interest is co-transfected into HEK-293T packaging cells along with Rep/Cap vector, which determines the serotype, and helper vector, which encodes for adenovirus genes (E4, E2A and VA). Viral particles are then harvested from cell culture medium, purified and concentrated. A qPCR-based approach is then used to measure AAV titer after virus DNA isolation. AAV titer and infection capacity is between adenovirus and lentivirus. However, AAV has the lowest induced immune response in vivo. Our AAV are bacteria, fungi, mycoplasma and endotoxin-free.
